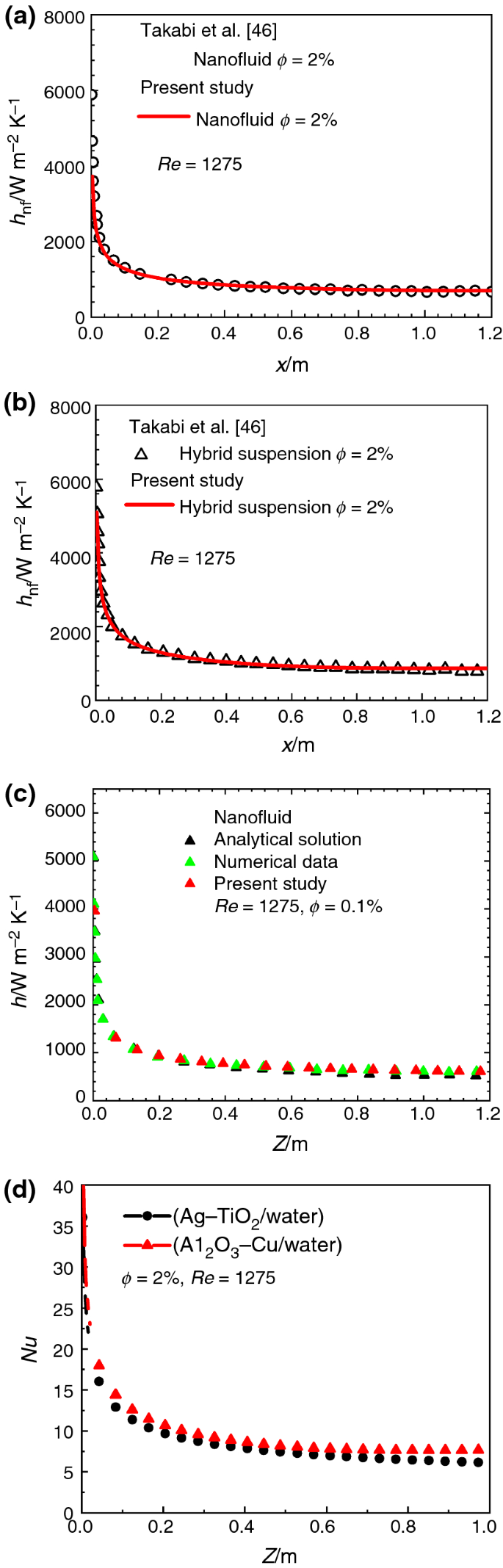
A System Delivers 1275 J Of Heat
A system delivers 1275 j of heat. A system delivers 1275 j of heat. A -2130 J B -420 J C 420 J D 2130 J E -1275 J. Calculate esys in j.
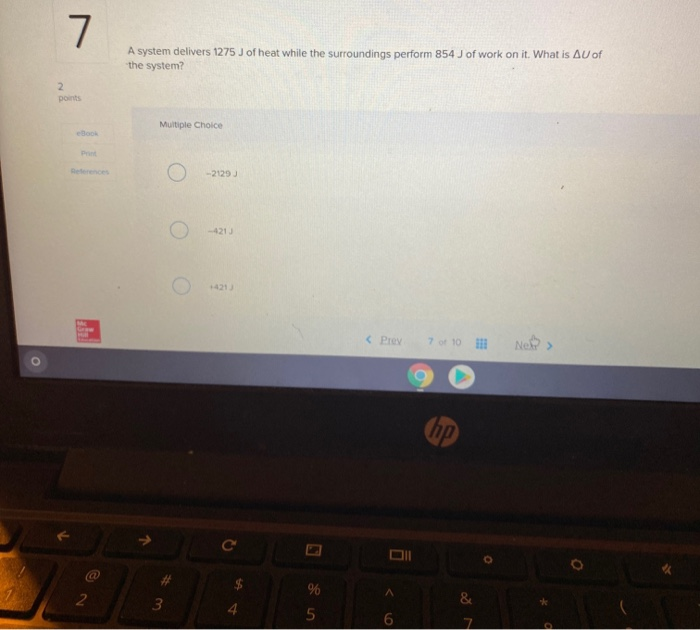
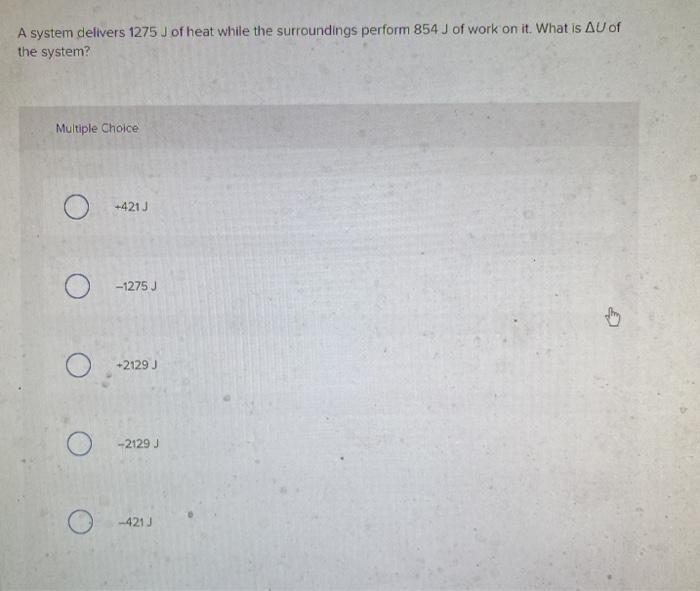
Calculate delta U in J. What is ΔE of the system. A system delivers 1275 J of heat while the surroundings perform 854 J of work on itWhat is ΔU of the system.
Calculate AU in J A -2130 J B -420J C 420J D 2130J E -1275 30 How many degrees of temperature rise will occur when a 250 g block of aluminum absorbs 100 kJ. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. The first law of thermodynamics says that the variation of internal energy of a system is given by.
Calculate ΔE in J. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. A -2130 J B -420 J C 420 J D 2130 J E -1275 J.
A device absorb 600 J of heat and work tantamount to 300 J on its surroundings. A 2130 J B 420 J C 420 J D 2130 J E 1275 J 32. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it.
A system delivers 225 J of heat to the surroundings while delivering 645 J of work. Calcium hydroxide which reacts with carbon dioxide to form calcium carbonate was used by the ancient Romans as. A system delivers 1275 j of heat.
-1275 J Determine The Quantity Of Ice Required To Absorb Exactly 50 KJ Of Energy When The Ice Warms From -500C To -100C specific Heat Ofice 206 Jg1 C-. Calculate Δ E in J-2130 J-420 J 420 J 2130 J-1275 J.
The first law of thermodynamics says that the variation of internal energy of a system is given by.
Calculate E In J. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. Calculate esys in j. Calculate ΔE in J. Calculate the change in the internal energy. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. A device absorb 600 J of heat and work tantamount to 300 J on its surroundings. A -2130 J B -420 J C 420 J D 2130 J E -1275 J. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it.
A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. Calculate delta U in J. -1275 J Determine The Quantity Of Ice Required To Absorb Exactly 50 KJ Of Energy When The Ice Warms From -500C To -100C specific Heat Ofice 206 Jg1 C-. A -2130 J B -420 J C 420 J D 2130 J E -1275 J. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it. A system delivers 1275 J of heat while the surroundings perform 854 J of work on it. A system delivers 1275 J of heat while the surroundings perform 855 J of work on it.
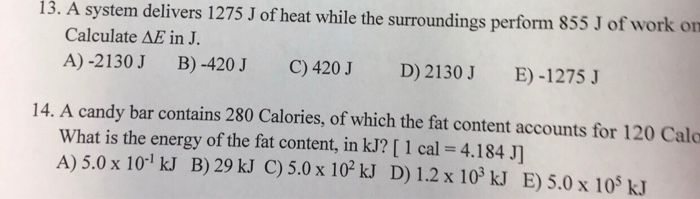

































Post a Comment for "A System Delivers 1275 J Of Heat"